In water salt actually splits into Na and Cl - ions which are very good at carrying current or the flow of electric charges. They react with acids to yield neutral salts.

Electrolysis Of Nacl Dilute Vs Concentrated
Chlorine and hydrogen are co-produced.

. Rosenmund Reduction Acyl chloride acid chloride is hydrogenated over catalyst palladium on barium sulphate. Two other useful chemicals are obtained during the process sodium hydroxide NaOH and hydrogen H. Given I 4A t 1 x 60 x.
This is known as Kolbes reaction. This reaction is called Rosenmund reduction. During an electrolysis of molten sodium chloride a 4A current is passed through electrodes for 1 hour.
The chemical change is one in which the substance loses or gains an electron oxidation or reduction. The term alkali is also applied to the soluble hydroxides of such alkaline-earth. When the membrane is not in place hypochlorites are formed.
The largest electrolyzers are of this type and have the greatest commercial reach Dincer and Acar 2014The cell consists of a pair of electrodes separated by a diaphragm that is filled with an alkaline solution typically potassium hydroxide in a concentration between 25 and 30. Alkaline water electrolysis AWE is the most mature and simplest method. It is an essential electrolyte located in all body fluids responsible for.
By NTA 98 Allen Ans. The process is fueled by electrolysis. Calculate the mass of sodium that is produced during this time.
The caustic soda solution may be clear or slightly cloudy in appearance depending on the cell process used. The process of electrolysis involves using an electric current to bring about a chemical change and make new chemicals. It can be done in the laboratory.
Table salt or sodium chloride NaCl is also a good additive to form electrolytes. Alkali any of the soluble hydroxides of the alkali metalsie lithium sodium potassium rubidium and cesium. Electrolysis process by which electric current is passed through a substance to effect a chemical change.
The chloride ion ˈ k l ɔːr aɪ d is the anion negatively charged ion Cl It is formed when the element chlorine a halogen gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Brine is a solution of sodium chloride NaCl and water H 2 O. If potassium chloride or calcium chloride is used instead potassium hydroxide and calcium hydroxide are produced.
Alkalies are strong bases that turn litmus paper from red to blue. 50 mL of 01 M CH. Chloride salts such as sodium chloride are often very soluble in water.
A 05 percent solution of potassium chloride was found to freeze at 024C. Nearest integer Molal depression constant for water is 180 K kg mol 1. The process is carried out in an electrolytic cell an apparatus consisting of positive and negative electrodes held apart and dipped into a solution containing positively and.
The electrolysis of brine is a large-scale process used to manufacture chlorine from salt. The percentage dissociation of potassium chloride is _____. And they are caustic and in concentrated form are corrosive to organic tissues.
And molar mass of KCl is 746 g mol 1 Official Ans. If it is heated chlorates are formed. OxyChem produces sodium hydroxide NaOH solution commonly known as caustic soda through the electrolysis of sodium chloride salt brine in diaphragm or membrane cells.
98 or 99 3. Phenol with sodium hydroxide gives sodium phenoxide ion which with carbon dioxide in acidic medium results hydroxybenzoic acid salicylic acid.

Electrolysis Of Sodium Chloride Membrane Chemistry Solutions
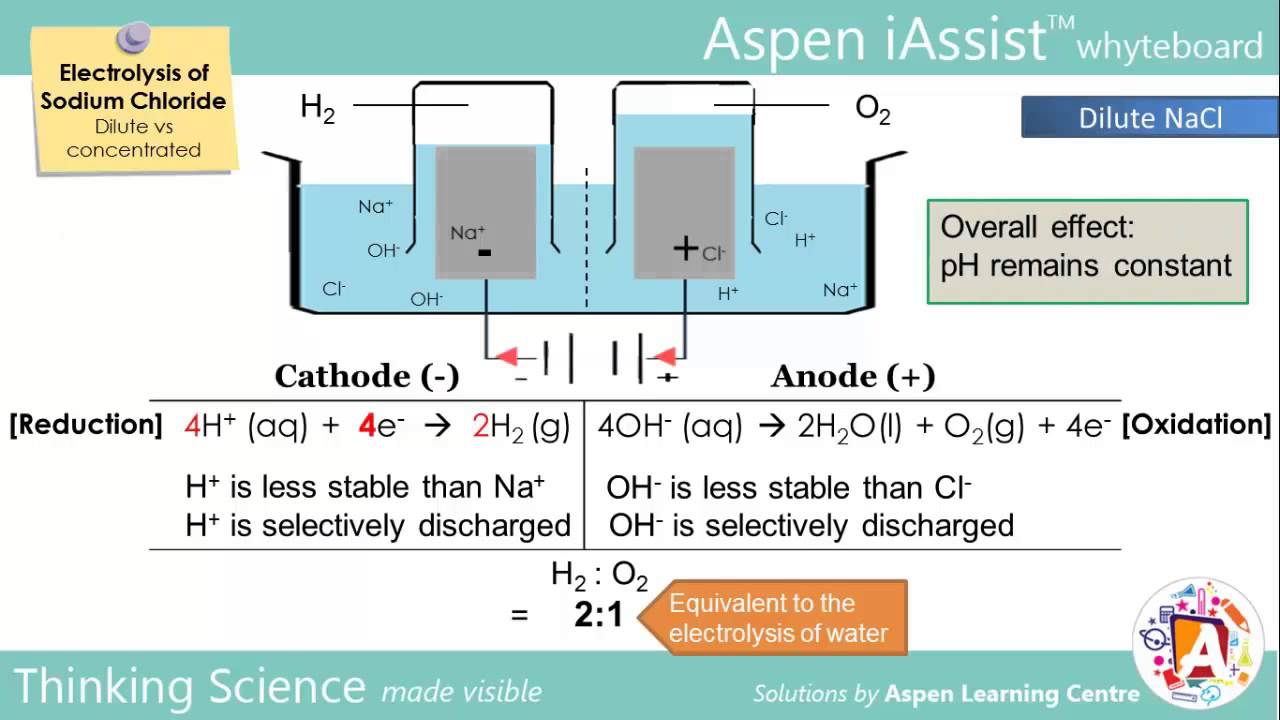
Electrolysis Of Nacl Dilute Vs Concentrated Chemistry E Book Ebook

What Is Electrolysis Electrolysis Of Molten Sodium Chloride Science Chemistry Chemistry Science Fair Projects

Electrolysis Of Molten Sodium Chloride Teaching Chemistry Chemistry Education Chemistry Classroom
0 Comments